
Identify the types of intermolecular forces experienced by specific molecules based on their structures. Describe the types of intermolecular forces possible between atoms or molecules in condensed phases (dispersion forces, dipole-dipole attractions, and hydrogen bonding). Appendix L: Standard Electrode (Half-Cell) Potentialsīy the end of this section, you will be able to:. Appendix K: Formation Constants for Complex Ions. Appendix I: Ionization Constants of Weak Bases. Appendix H: Ionization Constants of Weak Acids. Appendix G: Standard Thermodynamic Properties for Selected Substances. Appendix F: Composition of Commercial Acids and Bases. Appendix D: Fundamental Physical Constants. Appendix C: Units and Conversion Factors. #Examples of each dispersio forces free#
Second Law of Thermodynamics and Gibbs Free Energy. Application: Precipitation and Dissolution. Shifting Equilibria: LeChatelier’s Principle. Chemical Equilibria and Applications Toggle Dropdown Collision Theory and Factors Affecting Reaction Rates. Solutions and Colligative Properties Toggle Dropdown Chemical Bonding and Molecular Geometry Toggle Dropdown 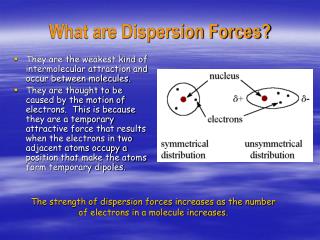
Thermochemical Guidelines, Enthalpy of Formation and Hess's Law.Solution Stoichiometry and Combustion Analysis.Writing and Balancing Chemical Equations.Stoichiometry of Chemical Reactions Toggle Dropdown Determining Empirical and Molecular Formulas.Composition of Substances and Solutions Toggle Dropdown Molecular and Ionic Compounds and Their Nomenclature.


Early Ideas and Evolution of Atomic Theory.Atoms, Molecules and Ions Toggle Dropdown Measurements and Uncertainty in Measurement.Classification, Physical and Chemical Properties.







 0 kommentar(er)
0 kommentar(er)
